Abstract
Background
Hyperleukocytosis can occur in acute leukemia at diagnosis or relapse, and leukostasis is more common in Acute Myeloid Leukemia (AML) than lymphoid leukemias due to larger blasts and increased adhesiveness. Patients presenting with hyperleukocytosis have early mortality rates of 20% during induction therapy and require prompt cytoreductive treatment (Oberoi et al. Leuk Res. 2014). The optimal cytoreductive technique is controversial with options including hydroxyurea, intensive chemotherapy, or leukapheresis. Due to the rarity of leukostasis and need for emergent treatment, randomized trials have not been performed. We previously investigated leukapheresis for this indication and found a 46.9% mortality by day 30 at our institution in patients with newly diagnosed AML (Hambley et al. Blood 2019). Subsequently, we have elected to use gemtuzumab ozogamicin (GO), an antibody-drug conjugate directed against CD33 approved for the treatment of adults with CD33+ AML, to manage hyperleukocytosis and leukostasis. We evaluated outcomes of patients receiving GO for this indication.
Methods
Demographic, clinical, laboratory, and outcome data were collected for AML patients treated with GO for cytoreduction at Johns Hopkins Hospital with a peak white blood cell (WBC) count greater than 50K/mm3. Demographic data consisted of age, sex, disease status (de novo presentation versus relapsed disease), ECOG performance status, APACHE II score, and Charlson Comorbidity Index (CCI) score. Clinical data incorporated CD33 status, karyotype, gene mutations, ELN risk stratification, and central nervous system (CNS) involvement. Laboratory data included peak WBC count, WBC count 72 hours after receiving GO, and laboratory markers of tumor lysis syndrome and disseminated intravascular coagulation within 72 hours of GO. Both toxicities pre-treatment and within 72 hours after treatment with GO were graded based on the Common Terminology Criteria for Adverse Events (CTCAE). The main outcomes recorded were response to induction and overall survival (OS).
Results
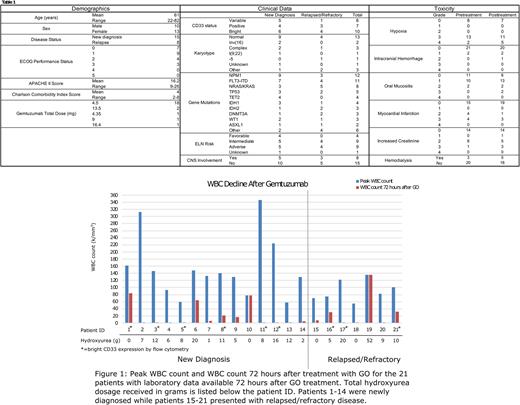
Demographics, clinical data, and toxicities are summarized in Table 1. Twenty-three patients received GO for cytoreduction from 2019 through 2021. Fifteen patients presented with newly diagnosed AML while 8 presented with relapsed disease. The blasts for all patients expressed CD33 with expression ranging from variable in 6 (26.1%), positive in 7 (30.4%), and bright in 10 (43.5%). For newly diagnosed patients, the ELN disease risk classification was favorable in 4 (26.7%), intermediate in 5 (33.3%), and adverse in 5 (33.3%). A significant portion (8/23) of patients had CNS involvement. Prior to treatment with GO, 15/23 (65.2%) patients had grade 3-4 hypoxia with 2 patients requiring intubation, 8/23 (34.8%) had evidence of myocardial damage, and 9/23 (39.1%) had acute kidney injury with 3 patients requiring dialysis. Most patients (18/23) received a single dose of 4.5mg GO. Patients also received hydroxyurea in 18/23 (78.3%) cases, receiving a median total dosage of 8 grams (range of 0-52g). Patients treated with GO had a median 96.9% reduction in WBC count 72 hours after treatment with a range of 0-99.7% (See Figure 1). The main grade 3-4 toxicities after GO included hypoxia and increased creatinine, which were often present prior to GO. Rasburicase was used for elevated uric acid in 13/23 (56.5%) cases. In the newly diagnosed cohort, 8/15 (53.3%) patients achieved a complete remission (CR) after induction with a 30-day OS of 86.7% and 1-year OS of 55%. Not surprisingly, the outcomes for the relapsed cohort were inferior with 2/8 (25.0%) achieving a CR, a 30-day OS of 75.0%, and a 1-year OS of 15%.
Conclusions
Gemtuzumab ozogamicin treatment effectively lowers WBC count in AML patients presenting with hyperleukocytosis and leukostasis, and 30-day mortality was lower than our experience with leukapheresis. Overall, GO was well-tolerated and provides another option for cytoreduction in patients with AML presenting with WBC counts greater than 50K/mm3.
Disclosures
Jain:Care Dx, Bristol Myers Squibb, Incyte, Abbvie, CTI, and Kite: Other: Advisory Board participation; CTI Biopharma, SyneosHealth, Incyte: Research Funding. Ghiaur:Scripps: Consultancy, Research Funding; Menarini Group: Consultancy, Research Funding; Syros Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Hourigan:TwinStrand Biosciences: Other; Qiagen: Other; Mission Bio: Other; Archer Diagnostics: Other; Sellas Life Sciences (Inst): Research Funding. DeZern:Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; CTI BioPharma: Consultancy, Honoraria; Syntrix Pharmaceuticals: Research Funding; GERON: Other: DSMB. Gojo:Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Research Support; Merck: Other: Research support; Amphivena: Other: Research Support; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Research support; Genentech: Other: Research support; Celgene: Other: Research support; Immunogen: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Certara: Membership on an entity's Board of Directors or advisory committees; Clearview: Membership on an entity's Board of Directors or advisory committees; Ono Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Johnson & Johnson: Membership on an entity's Board of Directors or advisory committees. Webster:Pfizer: Consultancy; Amgen: Consultancy. Levis:AbbVie, Amgen, Astellas, Bristol Myers Squibb, Daiichi-Sankyo, FujiFilm, Jazz Pharmaceuticals, and Menarini: Consultancy; Astellas, and FujiFilm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal